- Have any questions?
- 91-22-23726950
- 91-22-23774610

Resorcinol
May 10, 2019
Selenium Dioxide
May 10, 2019Saccharin
Muby Chemicals established in the year 1976, is pioneer in Manufacturing Chemicals for Oil and Gas Exploration, Hydraulic Fracturing (Fracking) and coiled tube Chemicals.Our advanced chemistry leading to an innovative and high-performance product range is coupled with effective on and off site management services.
We are manufacturer of Specialty chemicals, Pharmaceutical Excipients, Fragrance & Flavorchemicals in India, which are of IP, BP, USP, Ph. Eur., FCC or Food Grade, ACS, AR or Analytical Reagent Grade, LR or Laboratory Reagent Grade, Pure and Technical Grades of various chemicals.
Saccharin is primarily produced in two types i.e. soluble and insoluble saccharin. The soluble saccharin (in water) is called Sodium Saccharin whereas insoluble saccharin is called Insoluble Saccharin.
Saccharin is produced in two physical forms, viz granular and powder
Saccharin is used in a variety of industry such as food and beverage, personal care products, table top sweetener, electroplating brighteners, pharmaceuticals etc.
Insoluble Saccharin:
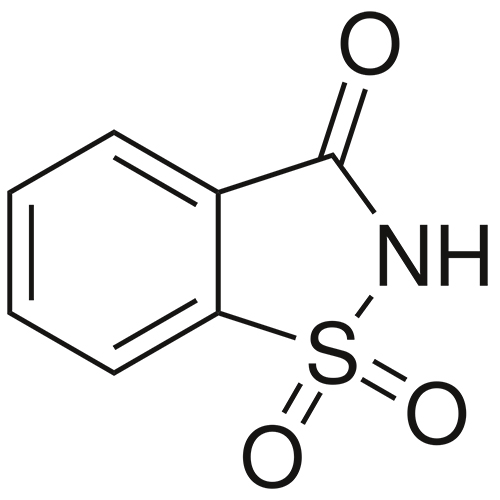
Saccharin is an artificial that is about 300–400 times as sweet as sucrose but has a bitter or after-taste. It is used to sweeten products such as drinks, candies, cookies, and medicines. Saccharin has no food energy and no nutritional value. It is safe to consume for individuals with diabetes.
General Specifications of Insoluble Saccharin
CAS NO: 81-07-2
Chemical Name: 1,2-benzisothiazolin-3(2H)-one 1,1-dioxide.
Molecular Formula: C7H5NO3S
Molecular Weight: 183.18
Description: White crystals or white, crystalline powder odourless or with a faint, aromatic odour.
Solubility: Sparingly soluble in boiling water and in ethanol (95%); slightly soluble in chloroform, in cold water and in ether. It dissolvers in dilute ammonia solution, in solutions of alkali hydroxides and carbonates.
Melting range: 226 -230C.
Loss on drying: Not more than-1.0%
Heavy metals: Not more than 20 ppm.
OTS Amide content: Not more than 200 ppm
Sulphated ash: Not more than 0.10%.
Assay: Not less than 98.0% and not more than 101.0% on dried substance.
Saccharin BP EP Ph Eur USP NF is also offered. As per the monograph of Saccharin BP EP Ph Eur its content analyses between 99 to 101% assay and meets the requirement of trace impurities specified in the monograph.
Saccharin USP NF
C7H5NO3S 183.19
1,2-Benzisothiazol-3(2H)-one, 1,1-dioxide.
1,2-Benzisothiazolin-3-one 1,1-dioxide [81-07-2].
Saccharin contains not less than 99.0 percent and not more than 101.0 percent of C7H5NO3S, calculated on the dried basis.
Packaging and storage: Preserve in well-closed containers. Store at room temperature.
Clarity of solution: [NOTE: The Test solution is to be compared to Reference suspension A and to water in diffused daylight 5 minutes after preparation of Reference suspension A.]
Hydrazine solution: Transfer 1.0 g of hydrazine sulfate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Allow to stand for 4 to 6 hours.
Methenamine solution: Transfer 2.5 g of methenamine to a 100-mL glass-stoppered flask, add 25.0 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension: [NOTE: This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use.] Transfer 25.0 mL of Hydrazine solution to the Methenamine solution in the 100-mL glass-stoppered flask. Mix, and allow to stand for 24 hours.
Opalescence standard: [NOTE: This suspension should not be used beyond 24 hours after preparation.] Transfer 15.0 mL of the Primary opalescent suspension to a 1000-mL volumetric flask, dilute with water to volume, and mix.
Reference suspensions: Transfer 5.0 mL of the Opalescence standard to a 100-mL volumetric flask, dilute with water to volume, and mix to obtain Reference suspension A. Transfer 10.0 mL of the Opalescence standard to a second 100-mL volumetric flask, dilute with water to volume, and mix to obtain Reference suspension B.
Test solution: Dissolve 5.0 g of test material in about 20 mL of a 200 g per L solution of sodium acetate, dilute with the same solution to 25 mL, and mix.
Procedure: Transfer a sufficient portion of the Test solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15 mm to 25 mm to obtain a depth of 40 mm.
Similarly transfer portions of Reference suspension A, Reference suspension B, water, and a 200 g per L solution of sodium acetate to separate matching test tubes. Compare the Test solution, Reference suspension A, Reference suspension B, water, and a 200 g per L solution of sodium acetate in diffused daylight, viewing vertically against a black. [NOTE—The diffusion of light must be such that Reference suspension A can readily be distinguished from water and that Reference suspension B can readily be distinguished from Reference suspension A.] The Test solution shows the same clarity as that of water, or the 200 g per L solution of sodium acetate, or its opalescence is not more pronounced than that of Reference suspension A.
Color of solution:
Standard stock solution: Combine 3.0 mL of ferric chloride, 3.0 mL of cobaltous chloride, 2.4 mL of cupric sulfate, and 1.6 mL of dilute hydrochloric acid (10 g per L).
Standard solution: [NOTE: Prepare the Standard solution immediately before use.] Transfer 1.0 mL of Standard stock solution to a 100-mL volumetric flask, dilute with dilute hydrochloric acid (10 g per L) to volume, and mix.
Test solution: Use the Test solution from Clarity of solution.
Procedure: Transfer a sufficient portion of the Test solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15 mm to 25 mm to obtain a depth of 40 mm.
Similarly transfer portions of the Standard solution, a 200 g per L solution of sodium acetate, and water to separate matching test tubes. Compare the Test solution, the Standard solution, a 200 g per L solution of sodium acetate, and water in diffused daylight, viewing vertically against a white background. The Test solution has the appearance of water or the 200 g per L solution of sodium acetate, or is not more intensely colored than the Standard solution.
Identification: Infrared Absorption 197K .
Melting range: between 226C and 230C.
Loss on drying: Dry it at 105C for 2 hours: it loses not more than 1.0% of its weight.
Readily carbonizable substances: Dissolve 200 mg in 5 mL of sulfuric acid (between 94.5% and 95.5% [w/w] of H2SO4), and keep at a temperature of 48C to 50C for 10 minutes: the solution has no more color than Matching Fluid A, when viewed against a white background.
Residue on ignition: not more than 0.2%. Ignition temperature: 600C ± 50C.
Heavy metals: 0.001%.
Limit of toluenesulfonamides:
Internal standard solution: Dissolve 25 mg of caffeine in methylene chloride, and dilute with the same solvent to 100 mL.
Reference solution: Dissolve 20.0 mg of USP o-Toluenesulfonamide and 20.0 mg of USP p-Toluenesulfonamide in methylene chloride, and dilute with the same solvent to 100.0 mL. Dilute 5.0 mL of the solution with methylene chloride to 50.0 mL. Evaporate 5.0 mL of the final solution to dryness in a stream of nitrogen. Dissolve the residue in 1.0 mL of the Internal standard solution.
Test solution: Suspend 10.0 g of the substance to be examined in 20 mL of water, and dissolve using 5 mL to 6 mL of 10 N sodium hydroxide. If necessary, adjust the solution with 1 N sodium hydroxide or 1 N hydrochloric acid to a pH of 7 to 8, and dilute with water to 50 mL. Shake the solution with four quantities each of 50 mL of methylene chloride. Combine the lower layers, dry over anhydrous sodium sulfate, and filter. Wash the filter and the sodium sulfate with 10 mL of methylene chloride. Combine the solution and the washings, and evaporate almost to dryness in a water bath at a temperature not exceeding 40C. Using a small quantity of methylene chloride, quantitatively transfer the residue into a suitable 10-mL tube, evaporate to dryness in a stream of nitrogen, and dissolve the residue in 1.0 mL of the Internal standard solution.
Blank solution: Evaporate 200 mL of methylene chloride to dryness in a water bath at a temperature not exceeding 40C. Dissolve the residue in 1 mL of methylene chloride.
Chromatographic system: The gas chromatograph is equipped with a flame-ionization detector and contains a 0.53-mm × 10-m fused silica column, coated with G3 phase (film thickness 2 Gm). The injection port, column, and detector temperatures are maintained at about 250C, 180C, and 250C, respectively; and nitrogen is used as the carrier gas at a flow rate of about 10 mL per minute. The injector employs a split ratio of 1:2.
Procedure: Inject about 1 GL of the Reference solution. Adjust the sensitivity of the detector so that the height of the peak due to caffeine is not less than 50% of the full scale of the recorder. The substances are eluted in the following order: o-toluenesulfonamide, p-toluenesulfonamide, and caffeine. The test is not valid unless the resolution between the peaks due to o-toluenesulfonamide and p-toluenesulfonamide is at least 1.5. Inject about 1 GL of the Blank solution. In the chromatogram obtained, verify that there are no peaks with the same retention times as the internal standard, otoluenesulfonamide, and p-toluenesulfonamide. Inject about 1 GL of the Test solution and 1 GL of the Reference solution. If any peaks due to o-toluenesulfonamide, and p-toluenesulfonamide appear in the chromatogram obtained with the Test solution, the ratio of their areas to that of the internal standard is not greater than the corresponding ratio in the chromatogram obtained with the Reference solution (10 ppm of o-toluenesulfonamide and 10 ppm of p-toluenesulfonamide).
Limit of benzoate and salicylate: To 10 mL of a hot, saturated solution of it add ferric chloride, dropwise: no precipitate or violet color appears in the liquid.
Assay: Accurately weigh about 500 mg of Saccharin, dissolve in 40 mL of alcohol, add 40 mL of water, mix, add phenolphthalein, and titrate with 0.1 N sodium hydroxide. Perform a blank titration, if necessary, and make the appropriate correction. Each mL of 0.1 N sodium hydroxide is equivalent to 18.32 mg of C7H5NO3S.
Saccharin FCC Food Grade
o-Benzosulfimide; Gluside; 1,2-Benzisothiazole-3(2H)-one-1,1-dioxide
C7H5NO3S Formula wt 183.18
INS: 954 CAS: [81-07-2]
DESCRIPTION
Saccharin occurs as white crystals or as a white, crystalline powder. Its solutions are acid to litmus. One gram is soluble in 290 mL of water at 25C, in 25 mL of boiling water, and in 30 mL of alcohol. It is slightly soluble in chloroform and in ether, and it is readily dissolved by dilute solutions of ammonia, solutions of alkali hydroxides, or solutions of alkali carbonates with the evolution of carbon dioxide.
Function: Nonnutritive sweetener.
REQUIREMENTS
Identification:
A. Dissolve about 100 mg of sample in 5 mL of a 1:20 solution of sodium hydroxide, evaporate the mixture to dryness and gently fuse the residue over a small flame until ammonia no longer evolves. After the residue has cooled, dissolve it in 20 mL of water, neutralize the solution with 2.7 N hydrochloric acid, and filter. Add 1 drop of ferric chloride to the filtrate. A violet color appears.
B. Mix 20 mg of sample with 40 mg of resorcinol, cautiously add 10 drops of sulfuric acid, and heat the mixture in a liquid bath at 200C for 3 min. After cooling, add 10 mL of water and an excess of 1 N sodium hydroxide. A fluorescent green liquid results.
Assay: Not less than 98.0% and not more than 101.0% of C7H5NO3S after drying.
Benzoic and Salicylic Acids: Passes test.
Lead: Not more than 2 mg/kg.
Loss on Drying: Not more than 1%.
Melting Range: Between 226° and 230C.
Readily Carbonizable Substances: Passes test.
Residue on Ignition: Not more than 0.2%.
Selenium: Not more than 0.003%.
Toluenesulfonamides: Not more than 0.0025%.