- Have any questions?
- 91-22-23726950
- 91-22-23774610

Stannous Chloride Dihydrate
May 7, 2019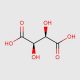
Tartaric Acid
May 7, 2019Stannous Fluoride
Muby Chemicals established in the year 1976, is pioneer in Manufacturing Chemicals for Oil and Gas Exploration, Hydraulic Fracturing (Fracking) and coiled tube Chemicals.Our advanced chemistry leading to an innovative and high-performance product range is coupled with effective on and off site management services.
We are manufacturer of Specialty chemicals, Pharmaceutical Excipients, Fragrance & Flavorchemicals in India, which are of IP, BP, USP, Ph. Eur., FCC or Food Grade, ACS, AR or Analytical Reagent Grade, LR or Laboratory Reagent Grade, Pure and Technical Grades of various chemicals.
Tin (II) fluoride SnF2, commercially referred to commercially as stannous fluoride. It is a colorless solid used as an ingredient in toothpastes that are typically more expensive than those that use sodium fluoride. Stannous fluoride converts the calcium mineral apatite into fluorapatite, which makes tooth enamel more resistant to bacteria-generated acid attacks. In toothpastes containing calcium minerals, sodium fluoride becomes ineffective over time, while stannous fluoride remains effective in strengthening tooth enamel.
SnF2 acts as a Lewis acid.
SnF2 is a reducing agent.
Stannous Fluoride USP Grade
SnF2 — 156.71
Tin fluoride (SnF2) [7783-47-3].
Stannous Fluoride contains not less than 71.2 percent of stannous tin (Sn ++), and not less than 22.3 percent and not more than 25.5 percent of fluoride (F), calculated on the dried basis.
Identification—
A: To 5 mL of a solution (1 in 100) in a test tube add 2 mL of calcium chloride TS: a fine, white precipitate of calcium fluoride is formed.
B: Mix on a spot plate 2 drops of a solution (1 in 100) with 2 drops of silver nitrate TS: a brown-black precipitate is formed.
C: Add 1 drop of a solution (1 in 100) to 2 drops of mercuric chloride: a white, silky precipitate is formed. On further addition of the solution (1 in 100), a brown-black precipitate is formed.
pH: between 2.8 and 3.5, in a freshly prepared 0.4% solution.
Loss on drying: Dry it at 105C for 4 hours: it loses not more than 0.5% of its weight.
Water-insoluble substances: Transfer about 10 g, accurately weighed, to a 400-mL plastic beaker, add 200 mL of water, and stir with a plastic rod for 3 minutes, or until no more solid dissolves. Filter through a tared filtering crucible, and wash thoroughly, first with ammonium fluoride solution (1 in 100), then with water. [NOTE—Prepare and use the filtering crucible in a well-ventilated hood.] Dry the residue at 105C for 4 hours, cool, and weigh: the weight of the residue does not exceed 0.2%.
Antimony: —
Rhodamine B solution: Dissolve 20 mg of rhodamine B in 200 mL of 0.5 N hydrochloric acid.
Standard preparation: Transfer 55.0 mg of antimony potassium tartrate, accurately weighed, to a 200-mL volumetric flask, dissolve in water, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 500-mL volumetric flask, add 6 N hydrochloric acid to volume, and mix.
Test preparation: Transfer 1.0 g of Stannous Fluoride, accurately weighed, to a 50-mL volumetric flask, add 6 N hydrochloric acid to volume, and mix.
Procedure: Pipet 5 mL each of the Standard preparation and the Test preparation into separate 125-mL separators, add 15 mL of hydrochloric acid and 1 g of ceric sulfate, and allow to stand for 5 minutes, with occasional shaking. Add 500 mg of hydroxylamine hydrochloride, and shake for 1 minute. Pipet 15 mL of isopropyl ether into the mixture, shake for 30 seconds, add 7 mL of water, and mix. Cool in a water bath at room temperature for 10 minutes, shake for 30 seconds, allow the layers to separate, and discard the aqueous phase. Add 20 mL of Rhodamine B solution, shake for 30 seconds, and discard the aqueous layer. Decant the ether layer from the top of the separator, and centrifuge, if necessary, to obtain a clear solution. Concomitantly determine the absorbances of the ether solutions from the Test preparation and the Standard preparation at the wavelength of maximum absorbance at about 550 nm, with a suitable spectrophotometer, using water as the blank: the absorbance of the Test preparation does not exceed that of the Standard preparation (0.005%).
Assay for stannous ion: —
0.1 N Potassium iodide-iodate: In a 1000-mL volumetric flask, dissolve 3.567 g of potassium iodate, previously dried at 110 to constant weight, in 200 mL of oxygen-free water containing 1 g of sodium hydroxide and 10 g of potassium iodide, dilute with oxygen-free water to volume, and mix. Standardize this solution by titrating a solution prepared from an accurately weighed quantity of reagent tin (Sn) and hydrochloric acid. Each mL of 0.1 N Potassium iodide-iodate is equivalent to 5.935 mg of Sn.
Procedure: Transfer about 250 mg of Stannous Fluoride, accurately weighed, to a 500-mL conical flask, and add 300 mL of hot, recently boiled 3 N hydrochloric acid. While passing a stream of an oxygen-free inert gas over the surface of the liquid, swirl the flask to dissolve the Stannous Fluoride, and cool to room temperature. Add 5 mL of potassium iodide, and titrate in an inert atmosphere with 0.1 N Potassium iodide-iodate, adding 3 mL of starch as the endpoint is approached. Each mL of 0.1 N Potassium iodide-iodate is equivalent to 5.935 mg of Sn++.
Assay for fluoride: —
[NOTE—Store all solutions, except Buffer solution, in plastic containers.]
Buffer solution: Dissolve 57 mL of glacial acetic acid, 58 g of sodium chloride, and 4 g of (1,2-cyclohexylenedinitrilo)tetraacetic acid in 500 mL of water. Adjust with 5 N sodium hydroxide to a pH of 5.25 ±0.25, dilute with water to 1000 mL, and mix.
Standard preparations: Dissolve an accurately weighed quantity of USP Sodium Fluoride quantitatively in water to obtain a solution containing 420 Cg per mL. Each mL of this solution (Standard preparation A) contains 190 Cg of fluoride ion (10 2 M). Transfer 25.0 mL of Standard preparation A to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution (Standard preparation B) contains 19 Cg of fluoride ion per mL (10 3 M). Transfer 25.0 mL of
Standard preparation B to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution (Standard preparation C) contains 1.9 Cg of fluoride ion per mL (10 4 M).
Assay preparation: Transfer to a 250-mL volumetric flask about 100 mg of Stannous Fluoride, accurately weighed. Add 50 mL of water, mix vigorously for 5 minutes, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL volumetric flask, dilute with water to volume, and mix.
Procedure: Pipet 20 mL of each Standard preparation and of the Assay preparation into separate plastic beakers each containing a plastic-coated stirring bar. Pipet 20 mL of Buffer solution into each beaker. Concomitantly measure the potentials, in mV, of the solutions from the Standard preparations and of the solution from the Assay preparation, with a pH meter capable of a minimum reproducibility of ±0.2 mV and equipped with a fluoride-specific ion-indicating electrode and a calomel reference electrode. [NOTE—When taking measurements, immerse the electrodes in the solution, stir on a magnetic stirrer having an insulated top until equilibrium is attained (1 to 2 minutes), and record the potential. Rinse and dry the electrodes between measurements, taking care to avoid damaging the crystal of the specific-ion electrode.] Plot the logarithms of the fluoride-ion concentrations, in Cg per mL, of the Standard preparations versus potential, in mV. From the measured potential of the Assay preparation and the standard reponse line, determine the concentration, C, in Cg per mL, of fluoride ion in the Assay preparation: Calculate the percentage of fluoride (F) in the portion of Stannous Fluoride taken by the formula:
125C / W
in which C is the determined concentration of fluoride, in Cg per mL, in the Assay preparation, and W is the weight, in mg, of Stannous Fluoride taken.
For Original Monographs of IP Indian Pharmacopoeia BP British Pharmacopoeia USP US Pharmacopoeia FCC Food Grade product, please check with the respective web-pages or books.




