- Have any questions?
- 91-22-23726950
- 91-22-23774610
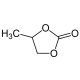
Propylene Carbonate
May 7, 2019
Sodium Acetate
May 7, 2019Selenious acid
Muby Chemicals established in the year 1976, is pioneer in Manufacturing Chemicals for Oil and Gas Exploration, Hydraulic Fracturing (Fracking) and coiled tube Chemicals.Our advanced chemistry leading to an innovative and high-performance product range is coupled with effective on and off site management services.
We are manufacturer of Specialty chemicals, Pharmaceutical Excipients, Fragrance & Flavorchemicals in India, which are of IP, BP, USP, Ph. Eur., FCC or Food Grade, ACS, AR or Analytical Reagent Grade, LR or Laboratory Reagent Grade, Pure and Technical Grades of various chemicals.
Selenous acid (or selenious acid) is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by (HO)2SeO.
The major use is in changing the color of steel, especially the steel in guns & razor blades the so-called “bluing” process which uses selenous acid. Some older were also made of blued steel. It is also used in darkening of copper, brass and bronze, producing a rich dark brown color.
Specifications of Commercial Pure Selenious Acid
CAS Number: [7783-00-8]
Molecular formula: H2SeO3
Molecular weight: 128.97
Description: White to off-white hygroscopic crystalline mass.
Residue on ignition: Max. 0.1 %.
Assay: Between 93.0% and 101.0%.
Selenious Acid USP Grade
H2SeO3 — 128.97
Selenium dioxide, monohydrated.
Selenious acid [7783-00-8].
Selenious Acid contains not less than 93.0 percent and not more than 101.0 percent of H2SeO3.
Identification
A: Dissolve about 50 mg in 5 mL of water, add about 100 mg of sodium bicarbonate, and mix: gas bubbles develop.
B: Dissolve about 50 mg in 5 mL of 0.1 N hydrochloric acid, and add about 50 mg of stannous chloride: a curdy tan-orange precipitate is formed.
Tests
Residue on ignition: not more than 1.0 mg, from 10.0 g (0.01%).
Insoluble matter: Dissolve 1 g in 5 mL of water: it dissolves completely and the solution is clear.
Selenate and sulfate: Dissolve about 0.5 g in 10 mL of water, add 0.1 mL of hydrochloric acid and 1 mL of barium chloride, and mix: no turbidity or precipitate is formed in 10 minutes.
Assay: Transfer about 100 mg of Selenious Acid, accurately weighed, to a suitable glass-stoppered flask, and dissolve in 50 mL of water. Add 10 mL of potassium iodide solution (3 in 10) and 5 mL of hydrochloric acid, mix, insert the stopper in the flask, and allow to stand for 10 minutes. Add 50 mL of water and 3 mL of starch, and titrate with 0.1 N sodium thiosulfate until the solution is colorless, then titrate with 0.1 N iodine VS to a blue endpoint. Subtract the volume of 0.1 N iodine from the volume of 0.1 N sodium thiosulfate to obtain the volume of 0.1 N sodium thiosulfate equivalent to selenious acid. Each mL of 0.1 N sodium thiosulfate is equivalent to 3.225 mg of H2SeO3.
For Original Monographs of IP Indian Pharmacopoeia BP British Pharmacopoeia USP US Pharmacopoeia FCC Food Grade product, please check with the respective web-pages or books.




